Characterization of Protein Aggregates and Other Particles in Biopharmaceuticals
Particulates are ubiquitous in parenteral drug products and remain a concern throughout their development and production. These particles must be monitored to satisfy USP particle reporting requirements (e.g. USP <788>). Furthermore, in biopharmaceuticals such as protein therapeutics, these particles have been associated with adverse impacts on the efficacy and safety of the product. FDA regulations strongly recommend in-depth characterization of the quantity and types of particles found in biotherapeutics.
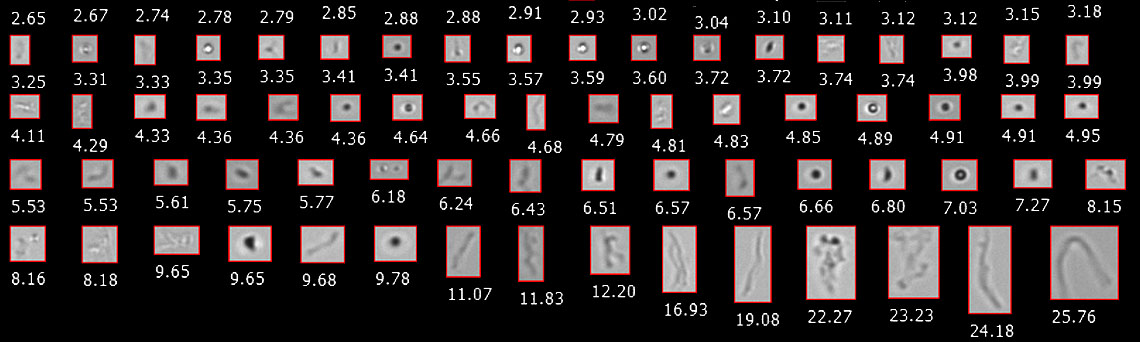
Light Obscuration (LO) is the primary compendial technique authorized by USP to monitor subvisible particles (i.e. particles 2-100 μm in diameter). While LO is effective for counting and sizing opaque particles, it is less effective at analyzing particles in biotherapeutics like aggregates of the active pharmaceutical ingredient (API) which are often highly translucent.
Flow imaging microscopy (FIM) is a USP-recognized orthogonal technique (via USP <1788>) to complement LO and other compendial techniques for particle counting and sizing. FIM instruments like FlowCam capture light microscopy images of particles as they flow through a flow cell. This method of particle detection can quickly count and size particles in a liquid sample. Since a digital image is recorded for each particle, FIM can also be used to capture particle morphology information not available from LO measurements.
Download the new application note from Yokogawa Fluid Imaging Technologies, Inc. to learn more about how FlowCam 8100 can accurately count and image particles in biotherapeutics.
Characterization of Protein Aggregates and Other Particles in Biopharmaceuticals